Astronomy 102 – Spring 2000
Tutorial #4: Nucleosynthesis of Elements in Stars
Including Answers
As the core of main sequence stars hydrogen is fused into
helium. Main sequence stars that are much more massive than the sun fuse
hydrogen into helium. Then, after the hydrogen is exhausted and the core
contracts some, helium is fused into carbon and oxygen – the third and
forth most abundant elements in nature.
Please use the table in appendix 10 in RETU for finding
the names of the elements, but do not use it in calculations! (this table
averages out over various isotopes). Answer the following questions.
-
When 3 heliums (4.0026 a.m.u) join to make a carbon 12 atom
(12.000 a.m.u.), how many atomic mass units are "released" as energy? ________0.0078
a.m.u______
-
With 1 atomic mass unit = 1.66 x 10-27 kg, how
much mass in kg is released? _______1.3 x 10^(-29) kg_______
-
With E=mc^2, how much energy is released? _1.2 x 10^(-12)
Joule___________
-
After the helium is exhausted - the core contracts again.
Then carbon and oxygen fuse. Carbon with carbon, carbon with oxygen and
oxygen with oxygen. When two carbon atoms fuse, what element (23.96 a.m.u)
do they form? ____Magnesium (Mg)_
-
Is energy released in the process? (Don’t calculate it!)
__Yes, because 23.96 < 12.000+12.000__
-
When two oxygens fuse, what element (31.96 a.m.u.) do they
form? ____Sulfur (S)___
-
When an oxygen nucleus fuses with a carbon nucleus they form
silicon (27.97 a.m.u.), the most abundant element on our earth. After the
carbon and oxygen are exhausted and the core contracts again one possible
fusion is of two silicone. In that process 2 protons become neutrons. What
is the product of those (55.5 a.m.u.)? Is energy released in this fusion?
__Iron (FE). Yes, 55.5 a.m.u. < 27.97 a.m.u. + 27.97 a.m.u_____
-
When the fusion of silicon, and other elements of that stage
stops, the core contracts again. When the element from 7 fuses with its
own kind and an extra proton, and 5 protons become neutrons, what element
(112.4 a.m.u.) may they form? ___Cadmium____
-
Is energy released in that process? ____No. 112.4 > 55.5+55.5____
-
Why is there no energy released in the process? (Remember
that the strong "Velcro" force acts over a very short range, whereas the
repulsion of positives by positives works over a very long range.) __Energy
was released in the previous fusion events because of the strong force
pull by protons & neutrons on each other. When we place many protons
together ALL OF THEM still push on each other, but only the ones that are
"touching" pull each other. Eventually the push by the electric force is
larger than the strong-force pull, and we do not gain energy by joining
more protons to a larger nucleus._(This is actually the basis for nuclear
FISSION of nuclear power stations: you take a large nucleus, say Uranium
(238 a.m.u.) and break it into smaller atoms in which the pull by the strong
force is stronger than the push by the other protons. In this way energy
is GAINED._)
The element you found in 10 never forms! The element that
you found in 7 is the last one to form in the cores of stars!
-
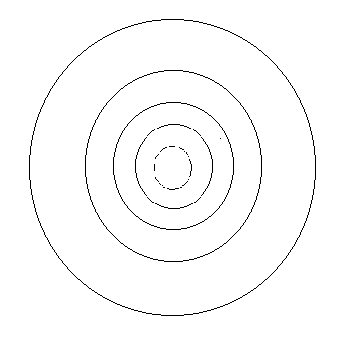 In the figure
below, better known as the "shell model" of nucleosynthesis in heavy stars,
fill in the names of elements according to where they are most abundant:
Hydrogen in the outer shell, then helium, than carbon and oxygen, then
silicone, magnesium and sulfur, then iron.
In the figure
below, better known as the "shell model" of nucleosynthesis in heavy stars,
fill in the names of elements according to where they are most abundant:
Hydrogen in the outer shell, then helium, than carbon and oxygen, then
silicone, magnesium and sulfur, then iron.
 In the figure
below, better known as the "shell model" of nucleosynthesis in heavy stars,
fill in the names of elements according to where they are most abundant:
Hydrogen in the outer shell, then helium, than carbon and oxygen, then
silicone, magnesium and sulfur, then iron.
In the figure
below, better known as the "shell model" of nucleosynthesis in heavy stars,
fill in the names of elements according to where they are most abundant:
Hydrogen in the outer shell, then helium, than carbon and oxygen, then
silicone, magnesium and sulfur, then iron.