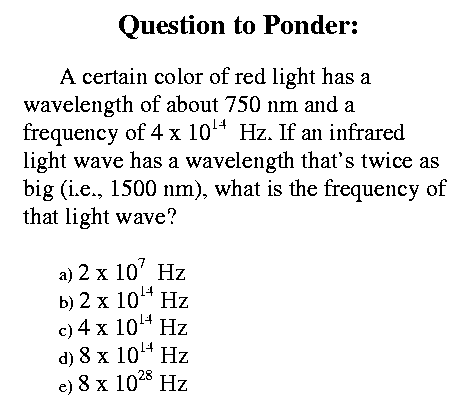
In Class:
-----------------------------------------------------
review:
light is a wave of electromagnetic energy
can be made by wiggling electrical charges
charges are everywhere, so that's not a problem
propagates through empty space by "bootstapping" itself
changing electric and magnetic fields
light is freely travelling energy
-------------------------------------------------------
If only it were that simple.
-- enter Albert Einstein
-- photoelectic effect
-- blasting electrons off of a metal surface
-- need a certain amount of energy to do it
e- like to stay on the metal
-- use light to put the energy need it
-- certain colors of light could do it
-- blue
-- other colors can't
-- red
-- light intensity didn't matter
-- very strange for a wave
-- shouldn't have to change
wavelength
-- increasing amplitude does not
increase energy
deposition
-- light must come in compartmentalized packets
whose energy is dependent on
wavelength
-- more intensity --> more packets, but
energy/packet is dependent on wavelength
-- back to a particle picture
red light consists of packets of low energy
delivered to matter in small bits
a bright light can still deliver a lot of energy
but it's a little bit at a time
blue light consists of packets of high energy
delivered to matter in large bits
a faint light may have very few packets
but each packet still carries a lot of punch
Analogy: rain
red light is like a fine mist
each drop is tiny -- like each red photon
however, if the mist is thick enough (i.e., high intensity)
you'll still get really wet.
blue light is like big raindrops(like just as a Tstorm starts)
each drop is big -- like each blue photon
each drop makes a big impact -- splash!
a lot of water can be delivered with just a few drops
either way, a lot of water (ie, energy) can be delivered,
even though the drop size (ie. photon energy)
is very different for the two types of rain.
Weird result: E = h nu = h c / lambda
h= 6.626 e -34
photon energy is quantized, and depends on wavelength
For blue light (400 nm), each photon contains
6.626e-34 * 3e8 / 400e-9
= 5e-19 J
want 1e-19 J? can't get it with blue light.
smallest bit, the photon, contains 5e-19 J.
you can have 10 e-19 J; that's two photons
you can have any multiple of this energy
So what does this mean?
- every time blue light is absorbed by a material, it is absorbed in
bits of 5e-19 J each, no less, no more
red photons deposit only 3e-19 J each
apparently, you need more than 3e-19 J to get the electron off
the metal.
--> this is why red light doesn't blast e- off.
- this also means that you need to gather together 5e-19 J of energy if
you want to make a blue photon.
can't make one with less.
-- has some interesting implications
-- if you have only so much energy, you can only create
certain photons
-- need to concentrate enough energy to make high
energy photons
in general,
--> high energy processes (e.g., hot processes; explosions)
-- produce high energy photons -- xrays, uv
--> low energy processes (e.g., us, cold interstellar clouds)
-- produce low energy photons -- ir, radio
there are exceptions to this rule, and we'll discuss those, too
-- detecting photons of different energies is different, too
-- visible light photons bounce off regular mirrors
-- collect starlight to analyze it
-- but xrays blast right through
-- need to make "grazing incidence" mirrors
Chandra X-Ray telescope --> web
|

![]()