 |
Astronomy 101 Problem Set #5 Solutions |  |
 |
Astronomy 101 Problem Set #5 Solutions |  |
Problem #1: Sunscreen performs a remarkable task for us. The little molecules in sunscreen absorb ultraviolet light which would otherwise impact your skin with the potential to break apart important biological molecules and substantially increase your chances of getting cancer. However, after they absorb the energy from these ultraviolet photons, the sunscreen molecules have to get rid of it. They do this by emitting infrared photons, which because of their much lower energy, are harmless to us. So in a real sense, sunscreen is a "wavelength converter," turning ultraviolet photons into infrared ones.
a) An ultraviolet photon has a wavelength of about 300 nm. Calculate the energy contained in one photon of this wavelength.
b) The molecules in your body are held together with chemical bonds whose typical binding strength is about 6 x 10-19 Joules. Another way to say this is that you can break these chemical bonds with 6 x 10-19 Joules of energy. If those bonds break or your chemistry is altered in some way, the damage to your system can be substantial, and might include creating cancers. Is the energy you found in part a) sufficient to potentially cause damage to your tissue?
c) Let's assume that the sunscreen converts the energy it receives when it absorbs one 300 nm photon into infrared photons with a wavelength of 2100 nm. Calculate the energy contained in one 2100 nm photon and determine if this type of photon is capable of doing damage to your tissue.
d) How many 2100 nm photons would a sunscreen molecule have to emit to get rid of the energy it collected when it absorbed one 300 nm photon?
Solution: Part a) is a straightforward application of the photon energy relationship:
Part b) just involves comparing your answer from part a) to the energy required to break typical biochemical bonds. Since 6.63 x 10-19 Joules > 6 x 10-19 Joules, it looks like these photons can do some damage to you.
Part c) is just a repeat of part a), only this time for a 2100 nm photon:
Part d) is an input-output problem. Energy comes in the form of an ultraviolet photon, and leaves in the form of infrared photons. If the sunscreen is supposed to do this many times to protect you from solar uv rays, it'll need to get rid of the energy it absorbs from each uv photon. So, energy in = energy out, right? So,
Problem #2: The filament in a regular incandescent lightbulb reaches a temperature of 2900 K when it's on.
a) Calculate the wavelength at which the blackbody spectrum from this filament is most intense.
b) What type of light (e.g., utraviolet, optical, infrared, radio) is a photon of this wavelength?
c) In light of your answer to part b), explain why incandescent light bulbs are not a very efficient way to produce optical light.
Solution: OK, let's start with Wien's Law, which is detailed on page 100 of your textbook:
In part c), I ask you to think a little about what this result means. If the blackbody spectrum peaks in the infrared, that means that most of the light from an incandescent light bulb comes out at these wavelengths, and not in the visible light range. It's still true that some light is emitted in the visible (if you don't think so, stare at a light bulb for a second), but a lot more infrared light is emitted. Consequently, a lot of the energy produced by a light bulb doesn't produce visible light at all. Incandescent light bulbs produce a lot more infrared light than they do visible light -- that's why they're so hot -- but that means a lot of the energy used by the bulb doesn't serve the bulb's intended purpose. The reality is that incandescent lights are better heaters than illuminators.
Problem #3: The Space Shuttle travels at a speed of 7700 m/s in orbit around the Earth at an altitude above the Earth's surface of 300 km. From this information, calculate the mass of the Earth.
Solution: This is a problem about gravity and circular motion. Since the Space Shuttle orbits in a circle, it must feel an inward pull -- if it didn't, it would zip off into space traveling in a straight line. The acceleration that the Shuttle experiences in its circular orbit is
OK, now let's use this expression. The only really tricky art of this problem lies in choosing the radius of the Space Shuttle's orbit. I've told you that the Shuttle orbits 300 km above our heads, but that does not mean that the radius of its orbit is 300 km. That would only be true if the Space Shuttle were circling our heads. In fact, the Shuttle circles the Earth, which is a larger larger than 300 km in radius. If you draw a picture, I think you'll find it pretty obvious that the radius of the Shuttle's orbit is equal to 300 km plus the radius of the Earth.
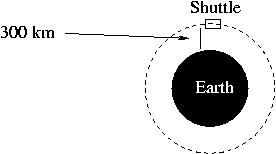
(If I had one wish for each and every one of you, it would be to get you to draw pictures for every single problem you encounter in this course.) So the radius of the Shuttle's orbit is:
Now we're ready to use the expression above to find the mass of the Earth:
